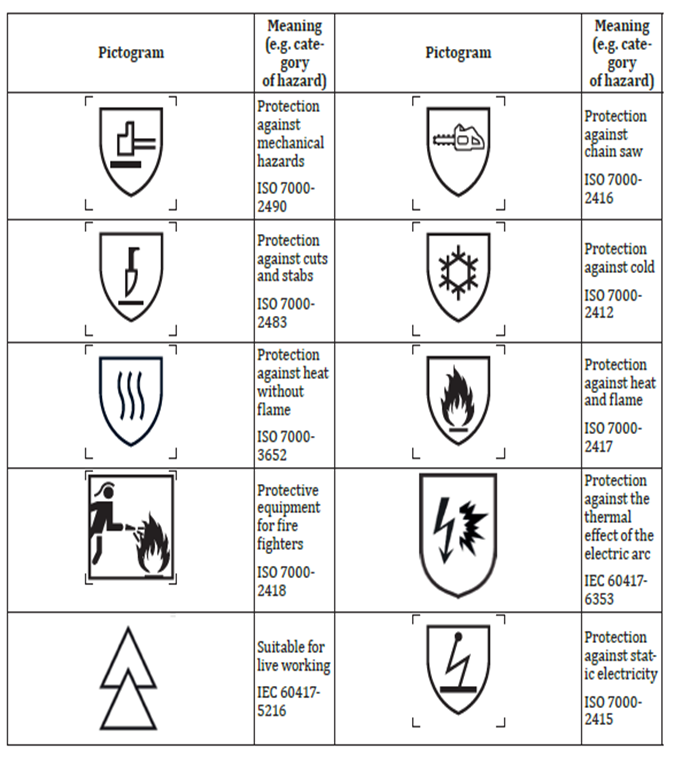
The TS EN ISO 21420 standard, which specifies general requirements and test methods for protective gloves, was amended 2024 July. This amendment further updates the TS EN ISO 2020 standard published in 21420 and includes significant improvements in certain areas.
Date Information in Cited Standards: With the amendment, date information has been added to the standards referred to in the TS EN ISO 21420 standard. For example, documents such as ISO 3071:2020, which is used to determine the pH values of gloves, and ISO 17075-1:2017, which is used to test the chromium VI content in leather-containing gloves, are now specified with date information. This change has been made to ensure that manufacturers and testing laboratories use accurate and up-to-date standards.
Chemical Content Limits: The amendment revises the limits for the chemical content of materials used in protective gloves. In particular, the chromium VI content in leather gloves has been set at 3.0 mg/kg and the amount of dimethylformamide (DMFa) in polyurethane (PU) gloves has been limited to 1000 mg/kg. The aim is to protect the health of users by ensuring that the chromium VI content is tested using ISO 17075-1:2017 or ISO 17075-2:2017 standards.
Electrostatic Properties: The testing of the electrostatic properties of protective gloves and appropriate labelling are also clarified in this amendment. With reference to the EN 16350:2014 standard, the evaluation of electrostatic properties and the correct labelling of gloves with these properties have been ensured.
Mechanical Strength and Tear Resistance: Within the scope of the amendment, the requirements for the resistance of protective gloves against mechanical risks have been revised. The methods used for tear resistance tests have been harmonised with the EN 388:2016+A1:2018 standard, aiming to provide safer products for users.
As USB Certification, we support you in the process of ensuring that your products comply with the TS EN ISO 21420 / A1: 2024 protective glove standard. This new amendment includes important updates for glove manufacturers and testing laboratories. It is of great importance that interested parties carefully review these changes and make the necessary arrangements.
Please contact us for more detailed information about this update or if you have any questions about the process.